
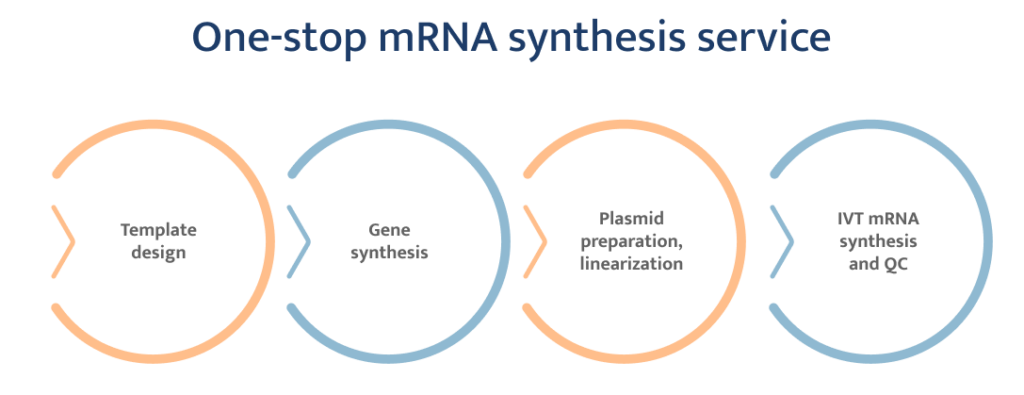
In Vitro Transcribed (IVT) mRNA provides a flexible and cost-effective platform to produce mRNA transcripts that can be used in many applications including therapeutics and vaccine development, gene editing and gene expression studies, antibody development, and more. IVT mRNAs can be transcribed from circular plasmid DNA, PCR products, and double-stranded oligos that contain the promoter sequences of phage RNA polymerases, most commonly T7.
Eurofins Genomics Blue Heron offers various services from DNA template options to IVT mRNA production. All our mRNA products are capped and polyadenylated to ensure stability and downstream application performance. Our standard QC and characterizations include size, purity, and integrity. Additional QC methods such as capping efficiency are available.


Quality Control and Specification of mRNA
| QC Item | Test Method | Specification | Standard | Upgrade |
| Appearance | Visual inspect | Clear, no visible particles | Yes | Yes |
| pH | pH meter | 5-8 | Yes | Yes |
| Concentration | Nanodrop | 0.5-1mg/ml | Yes | Yes |
| Purity | Nanodrop | 260/280 is 1.8-2.3 | Yes | Yes |
| RNA Length and Integrity | Gel electrophoresis | Expected fragment size observed | Yes | Yes |
| RNA Length and Integrity | Capillary electrophoresis | DV200 metric evaluation | No | Yes |
| Capping Efficiency | Enzyme digestion and gel electrophoresis | ≥ 90% | No | Yes |
| Endotoxin | Kinetic turbidimetric assay (KTA) – limulus amebocyte lysate (LAL) | < 10EU/mg | No | Yes |
How to Order
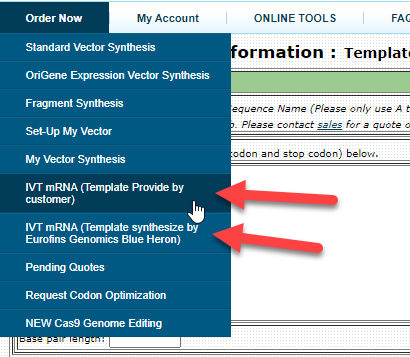
Online Ordering Available
Login to the ordering site and click the IVT mRNA links under Order Now.
Manual Request
Fill out the order form (right) and email it to sales@blueheronbio.com.
